The US Supreme Court on Friday protected access to the abortion pill, mifepristone, halting a lower court’s decision on the drug pending an appeal.
The SCOTUS decision allows mifepristone to remain available, subject to Food and Drug Administration (FDA) approval of the drug and follow-on actions that make the pill more easily accessible. This month, Texas District Attorney Judge Matthew Kaczmarik, an appointee of former President Donald Trump, ruled in favor of a November 2022 lawsuit filed by the anti-abortion organization Alliance for Hippocratic Medicine, which argued the FDA “never” had the right to approve. First use of mifepristone.
The Kacsmaryk decision is now pending appeal review by the 5th US Circuit Court of Appeals. President Joe Biden’s administration and a maker of mifepristone requested an emergency stay on Kaczmarik’s ruling after the federal government appealed the lower court ruling.
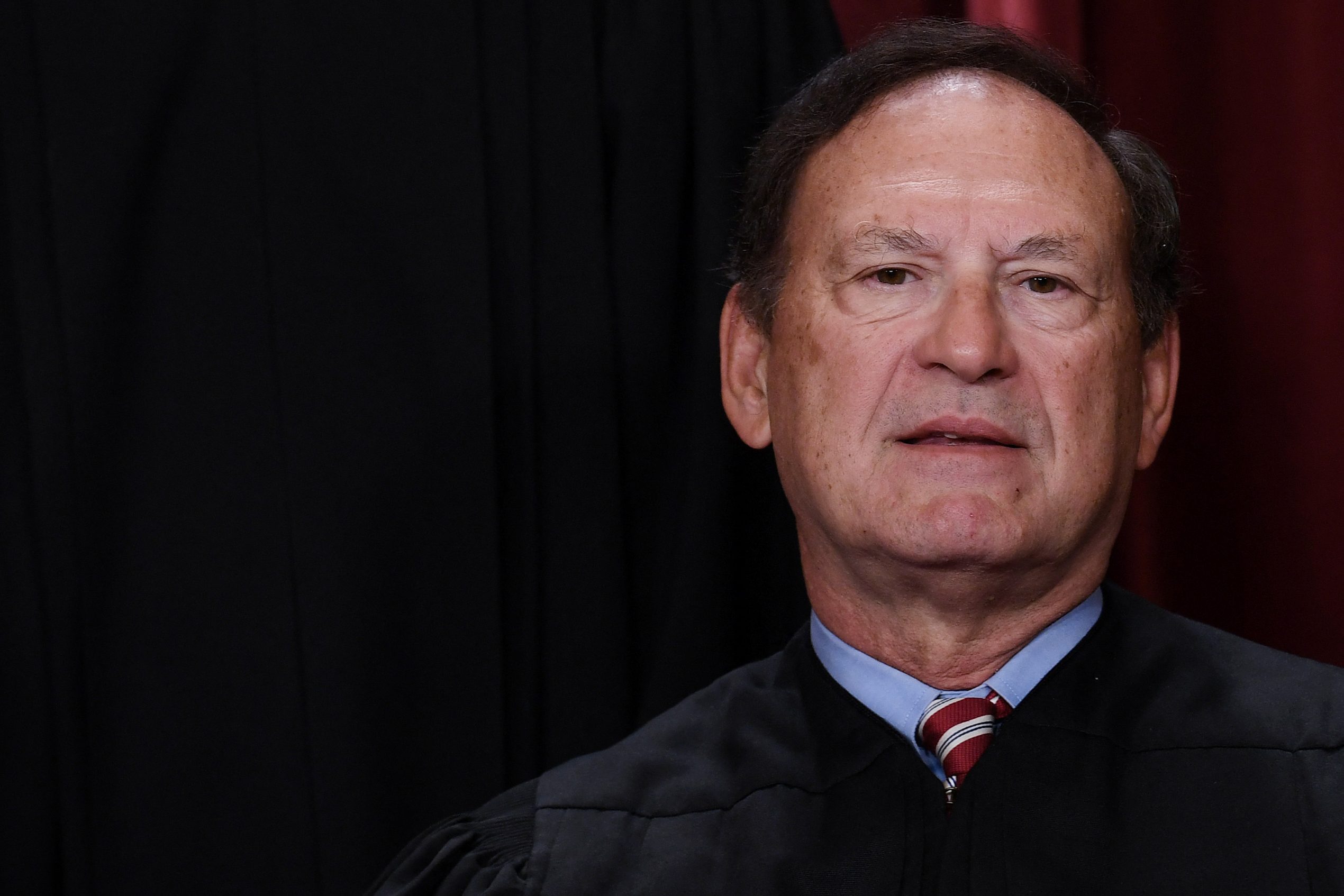
Supreme Court Justice Samuel Alito poses for an official photo on October 7, 2022 in Washington, DC. Alito, along with conservative Justice Clarence Thomas, on Friday sided with fellow justices who struck down a lower court ruling whose access would be restricted. For a widely used abortion drug.
OLIVIER DOULIERY/AFP/GETTY
of the Supreme Court one-paragraph judgment The unsigned was published and did not explain why the high court had decided to grant the Biden administration’s request. Conservative Justices Clarence Thomas and Samuel Alito publicly dissented from the decision, but the opinions of the other justices were not disclosed.
In his dissenting decision, Alito wrote that the requested stay could not be granted because the applicants “have not shown that they are likely to suffer irreparable harm” while the Fifth Court of Appeals hears arguments in the case.
Alito wrote, “As narrowed down by the Court of Appeals, this moratorium will not remove mifepristone from the market if we fail to broaden it.” “It would only restore the conditions that existed (and the government defended) from 2000 to 2016 under three presidential administrations.”
Mifepristone was first approved by the FDA in 2000 for medical termination of pregnancy during the first seven weeks of gestation. the decision was extendedHowever, in 2016 to allow the drug to be used up to 10 weeks of gestation.
Other decisions have since been made to expand access to the abortion pill, which is used in more than half of medically terminated pregnancies in the US. , the administration approved a rule change that allows retail pharmacies to offer the drug.
Alito also argued that since “the applicants’ Fifth Circuit appeal has been placed on the fast track, with oral arguments in 26 days, there is reason to believe they will get the relief they now seek – either the court appeals to or from this Court—in the near future if their arguments on the merits are persuasive.”
“At present, the applicants are not entitled to stay as they have not shown that they are likely to suffer irreparable harm in the interim,” he continued. “Applicants claim that regulatory ‘chaos’ would arise because of an alleged conflict between the relief granted in these cases and the relief granted by the decision of the United States District Court for the Eastern District of Washington.
“It is not clear whether there is actually a conflict because in these cases the relief is a stay, not an injunction, but even if there is a conflict, it should not be given any importance.”
At the same time that Kaczmarik took the side of an anti-abortion group, on April 7, Washington State District Judge Thomas O. A rival decision was made by Rice, an appointee of former President Barack Obama, who ordered that the FDA must keep mifepristone available. At least 17 Democratic-led states.
Friday’s decision is the second time SCOTUS has considered an attempt to restrict abortion access in the US in the past year. Conservative-led high court rules in June to overturn federal protections for abortion in landmark dobbs vs jackson Case.
Biden said in a statement It was published Friday evening that the Supreme Court’s decision to block their request “blocked from effect a lower court decision that undermined the FDA’s medical judgment and put women’s health at risk.” Used to give.”
“As a result of the Supreme Court’s stay, mifepristone remains available and approved for safe and effective use while we continue this fight in the courts,” read the statement. “I stand by the FDA’s evidence-based approval of mifepristone, and my administration will continue to defend the FDA’s independent, expert authority to review, approve and regulate a wide range of prescription drugs.”
Alliance Defending Freedom senior attorney Eric Baptiste, lead attorney representing the Alliance for Hippocratic Medicine and its fellow defendants, said in a statement statement shared with newsweek On Friday evening, the Supreme Court “decided to maintain the status quo” in its decision to grant a stay to the Biden administration.
“Our case for putting women’s health above politics continues to advance in the lower courts,” Baptiste said. “The FDA must answer for the harm it has done to the health of countless women and girls and to the rule of law, failing to study how dangerous the chemical abortion drug regimen is and unlawfully removing every meaningful protection, Even allowing mail-order abortions, we await a final outcome in this case that will hold the FDA accountable.”
newsweek has emailed the American Association of Pro-Life Obstetricians and Gynecologists for additional comment.